Urea Cycle Disorder Treatment Market to Grow by USD 215.4 Million (2024-2028), Driven by Rising Prevalence, with AI Redefining Market Landscape - Technavio
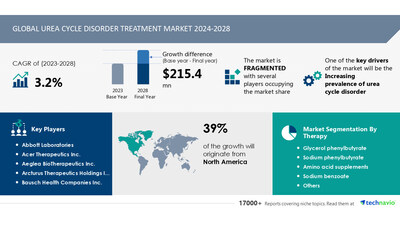
NEW YORK, Dec. 3, 2024 /PRNewswire/ -- Report with market evolution powered by AI - The global urea cycle disorder treatment market size is estimated to grow by USD 215.4 million from 2024-2028, according to Technavio. The market is estimated to grow at a CAGR of 3.2% during the forecast period. Increasing prevalence of urea cycle disorder is driving market growth, with a trend towards increasing growth potential in emerging economies. However, low access to specialized care poses a challenge. Key market players include Abbott Laboratories, Acer Therapeutics Inc., Aeglea BioTherapeutics Inc., Arcturus Therapeutics Holdings Inc., Bausch Health Companies Inc., Boehringer Ingelheim International GmbH, CAMP4, Dipharma SA, Eurocept B.V., Horizon Therapeutics Plc, Immedica Pharma AB, Medunik USA, Nestle SA, Orpharma Pty Ltd., Reckitt Benckiser Group Plc, Recordati S.p.A, RELIEF THERAPEUTICS Holding SA, Swedish Orphan Biovitrum AB, Synlogic Inc., and Ultragenyx Pharmaceutical Inc..
Key insights into market evolution with AI-powered analysis. Explore trends, segmentation, and growth drivers- View Free Sample PDF
Urea Cycle Disorder Treatment Market Scope | |
Report Coverage | Details |
Base year | 2023 |
Historic period | 2017 - 2021 |
Forecast period | 2024-2028 |
Growth momentum & CAGR | Decelerate at a CAGR of 3.2% |
Market growth 2024-2028 | USD 215.4 million |
Market structure | Fragmented |
YoY growth 2022-2023 (%) | 3.07 |
Regional analysis | North America, Europe, Asia, and Rest of World (ROW) |
Performing market contribution | North America at 39% |
Key countries | US, China, Germany, UK, and Canada |
Key companies profiled | Abbott Laboratories, Acer Therapeutics Inc., Aeglea BioTherapeutics Inc., Arcturus Therapeutics Holdings Inc., Bausch Health Companies Inc., Boehringer Ingelheim International GmbH, CAMP4, Dipharma SA, Eurocept B.V., Horizon Therapeutics Plc, Immedica Pharma AB, Medunik USA, Nestle SA, Orpharma Pty Ltd., Reckitt Benckiser Group Plc, Recordati S.p.A, RELIEF THERAPEUTICS Holding SA, Swedish Orphan Biovitrum AB, Synlogic Inc., and Ultragenyx Pharmaceutical Inc. |
Market Driver
The Urea Cycle Disorder (UCD) treatment market is experiencing significant growth due to increasing demand for advanced screening and diagnosis of diseases, rising healthcare expenditure, and the expansion of the healthcare industry in developing economies like India and China. These countries offer substantial market opportunities with large untapped potential for UCD product manufacturers. The healthcare sector in these regions is growing rapidly, driven by demographic shifts and rising healthcare expenditure. The number of hospitals in developing countries is increasing, leading to a higher demand for advanced diagnostic procedures. For instance, India and China had over 69,264 hospitals combined in 2020. This trend is expected to continue, increasing the demand for medical instruments, including those used in UCD treatment. The relatively low labor costs and abundance of raw material suppliers in these countries also attract vendors to establish a presence, making it an attractive market for both established players and new entrants.
The Urea Cycle Disorders (UCDs) market is experiencing significant growth, driven by the increasing prevalence of these metabolic disorders and the development of new treatments. According to a recent market report by Statpearls, key players in the market include Acer Therapeutics Inc, Thoeris GmbH, and Boehringer Ingelheim. Their offerings include Carglumic acid, OLPRUVA (sodium phenylbutyrate), and amino acid supplements like glycerol phenylbutyrate and sodium benzoate. The COVID-19 outbreak in Wuhan, China, has impacted the market, leading to disruptions in R&D spending in the pharmaceutical sector. However, the demand for oral and injectable treatments for UCDs, such as OTC Ornithine Transcarbamylase and AS Argininosuccinate Synthetase, remains strong. New treatments like CARBAGLU and RAVICTI are gaining popularity due to their effectiveness in managing conditions like hepatomegaly and hyperammonemia. The market is expected to continue growing, with biotech companies and research institutes in EU member states investing heavily in the development of new treatments for enzyme deficiency types of UCDs.
Request Sample of our comprehensive report now to stay ahead in the AI-driven market evolution!
Market Challenges
- Urea Cycle Disorders (UCDs) necessitate specialized treatment from experienced healthcare providers due to their complex nature. However, access to such care is limited, particularly in remote and underserved regions. The scarcity of expert healthcare providers and geographic barriers result in a low number of diagnosed cases and suboptimal treatment. UCD care involves multidisciplinary approaches, including dietary modifications, medications, and ammonia level monitoring. These complexities lead to delayed diagnoses, inadequate treatment, and minimal support for patients and their families. These challenges hinder the growth of the UCD treatment market during the forecast period.
- The Urea Cycle Disorders (UCDs) market faces challenges due to the complexities of treating these conditions. Key players like Acer Therapeutics Inc. Offer solutions with their products, such as Carglumic acid for hepatic UCDs and OLPRUVA (sodium phenylbutyrate) for UCDs with hyperammonemia. The COVID-19 outbreak in Wuhan, China, disrupted supply chains, affecting the market. Amino acid supplements, including glycerol phenylbutyrate and sodium benzoate, are also used. OTC ornithine transcarbamylase and AS argininosuccinate synthetase are available, but their efficacy varies. Market reports indicate a growing demand for oral and injectable treatments in hospital, retail, and online pharmacies. Companies like Thoeris GmbH, Boehringer Ingelheim, and Acer Therapeutics are investing in R&D to address enzyme deficiency types like ornithine transcarbamylase deficiency. The pharmaceutical sector and biotech companies, along with research institutes in EU member states, are collaborating to advance treatments. The route of administration and managing hepatomegality are crucial considerations.
Discover how AI is revolutionizing market trends- Get your access now!
Segment Overview
This urea cycle disorder treatment market report extensively covers market segmentation by
- 1.1 Glycerol phenylbutyrate
- 1.2 Sodium phenylbutyrate
- 1.3 Amino acid supplements
- 1.4 Sodium benzoate
- 1.5 Others
- 2.1 Oral
- 2.2 Injectables
- 3.1 North America
- 3.2 Europe
- 3.3 Asia
- 3.4 Rest of World (ROW)
1.1 Glycerol phenylbutyrate- Urea cycle disorders (UCDs) are metabolic conditions that affect the normal processing of ammonia in the body. Treatment for UCDs involves managing diet and medication. Nutritional therapy focuses on restricting protein intake and providing specialized formulas. Medications like sodium phenylbutyrate and glycine help convert ammonia to urea for elimination. Regular monitoring of blood ammonia levels is crucial for effective treatment. Pharmaceutical companies develop and market these essential medications, ensuring patients receive proper care and management for UCDs.
Download a Sample of our comprehensive report today to discover how AI-driven innovations are reshaping competitive dynamics
Research Analysis
The Urea Cycle Disorder (UCD) treatment market encompasses a range of protein-based therapies and amino acid supplements used to manage these rare metabolic disorders. UCDs, including ornithine transcarbamylase deficiency and hyperammonemia, impair the body's ability to process ammonia, leading to toxic buildup. R&D spending in the pharmaceutical sector and biotech companies, as well as research institutes in EU member states, is driving innovation in UCD treatments. Amino acid supplements, such as glycerol phenylbutyrate and sodium benzoate, are commonly used to provide alternative sources of nitrogen for the urea cycle. OTC ornithine transcarbamylase and argininosuccinate synthetase enzyme replacements are also available. These treatments are administered through various channels, including hospital pharmacies, retail pharmacies, and online pharmacies. Key therapies include oral and injectable forms of these supplements and enzyme replacements. Statpearls, a leading provider of medical information, reports ongoing research and development in this field. Acer Therapeutics Inc is one of the notable players focusing on UCD treatments, with a pipeline of potential therapies.
Market Research Overview
Urea Cycle Disorders (UCDs) are rare metabolic conditions caused by enzyme deficiencies, leading to an accumulation of ammonia in the blood. Protein breakdown in the body results in the formation of ammonia, which is normally converted into urea for elimination. However, in UCDs, this process is disrupted, leading to hyperammonemia and potential neurological damage. The pharmaceutical sector and biotech companies, including research institutes in EU member states, are investing significantly in R&D for UCD treatments. Ornithine transcarbamylase deficiency (OTCD) is a common UCD, and treatments include sodium phenylbutyrate (Thoeris GmbH, OLPRUVA) and glycerol phenylbutyrate. Other treatments include Carglumic acid, sodium benzoate, and amino acid supplements. The market for UCD treatments is growing, with oral and injectable medications available through hospital pharmacies, retail pharmacies, and online pharmacies. Companies like Boehringer Ingelheim, Acer Therapeutics, and Statpearls are developing new treatments for UCDs, including RAVICTI and CARBAGLU. The COVID-19 outbreak in Wuhan, China, has disrupted supply chains for some UCD treatments, leading to shortages in certain regions. UCDs are complex conditions that require ongoing management and monitoring, including regular blood tests for ammonia levels. Treatment may also involve dietary modifications and lifestyle changes. The market for UCD treatments is expected to continue growing as new treatments are developed and approved.
Table of Contents:
1 Executive Summary
2 Market Landscape
3 Market Sizing
4 Historic Market Size
5 Five Forces Analysis
6 Market Segmentation
- Therapy
- Glycerol Phenylbutyrate
- Sodium Phenylbutyrate
- Amino Acid Supplements
- Sodium Benzoate
- Others
- Route Of Administration
- Oral
- Injectables
- Geography
- North America
- Europe
- Asia
- Rest Of World (ROW)
7 Customer Landscape
8 Geographic Landscape
9 Drivers, Challenges, and Trends
10 Company Landscape
11 Company Analysis
12 Appendix
About Technavio
Technavio is a leading global technology research and advisory company. Their research and analysis focuses on emerging market trends and provides actionable insights to help businesses identify market opportunities and develop effective strategies to optimize their market positions.
With over 500 specialized analysts, Technavio's report library consists of more than 17,000 reports and counting, covering 800 technologies, spanning across 50 countries. Their client base consists of enterprises of all sizes, including more than 100 Fortune 500 companies. This growing client base relies on Technavio's comprehensive coverage, extensive research, and actionable market insights to identify opportunities in existing and potential markets and assess their competitive positions within changing market scenarios.
Contacts
Technavio Research
Jesse Maida
Media & Marketing Executive
US: +1 844 364 1100
UK: +44 203 893 3200
Email: media@technavio.com
Website: www.technavio.com/
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/urea-cycle-disorder-treatment-market-to-grow-by-usd-215-4-million-2024-2028-driven-by-rising-prevalence-with-ai-redefining-market-landscape---technavio-302320517.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/urea-cycle-disorder-treatment-market-to-grow-by-usd-215-4-million-2024-2028-driven-by-rising-prevalence-with-ai-redefining-market-landscape---technavio-302320517.html
SOURCE Technavio