Companion Diagnostics Market to grow by USD 28.98 Billion (2024-2028), driven by personalized medicine adoption, with AI powering market evolution - Technavio
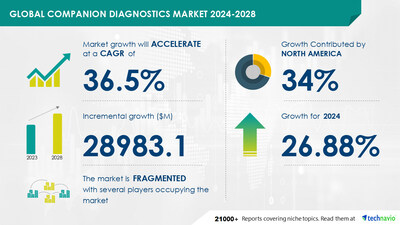
NEW YORK, Dec. 4, 2024 /PRNewswire/ -- Report on how AI is driving market transformation - The global companion diagnostics market size is estimated to grow by USD 28.98 billion from 2024-2028, according to Technavio. The market is estimated to grow at a CAGR of over 36.5% during the forecast period. Rising use of personalized medicine is driving market growth, with a trend towards rising occurrence of breast cancer. However, limited sustainability of smaller vendors poses a challenge. Key market players include Abbott Laboratories, Abnova Corp., Agilent Technologies Inc., Amoy Diagnostics Co. Ltd., ARUP Laboratories, Bayer AG, BioGenex Laboratories Inc., bioMerieux SA, F. Hoffmann La Roche Ltd., Guardant Health Inc., Illumina Inc., Invivoscribe Inc., Liquid Biotech USA Inc., Myriad Genetics Inc., NG Biotech, QIAGEN NV, Quest Diagnostics Inc., Siemens AG, Sysmex Corp., and Thermo Fisher Scientific Inc..
Key insights into market evolution with AI-powered analysis. Explore trends, segmentation, and growth drivers- View Free Sample PDF
Companion Diagnostics Market Scope | |
Report Coverage | Details |
Base year | 2023 |
Historic period | 2018 - 2022 |
Forecast period | 2024-2028 |
Growth momentum & CAGR | Accelerate at a CAGR of 36.5% |
Market growth 2024-2028 | USD 28983.1 million |
Market structure | Fragmented |
YoY growth 2022-2023 (%) | 26.88 |
Regional analysis | North America, Europe, Asia, and Rest of World (ROW) |
Performing market contribution | North America at 34% |
Key countries | US, UK, Germany, Japan, and France |
Key companies profiled | Abbott Laboratories, Abnova Corp., Agilent Technologies Inc., Amoy Diagnostics Co. Ltd., ARUP Laboratories, Bayer AG, BioGenex Laboratories Inc., bioMerieux SA, F. Hoffmann La Roche Ltd., Guardant Health Inc., Illumina Inc., Invivoscribe Inc., Liquid Biotech USA Inc., Myriad Genetics Inc., NG Biotech, QIAGEN NV, Quest Diagnostics Inc., Siemens AG, Sysmex Corp., and Thermo Fisher Scientific Inc. |
Market Driver
The Companion Diagnostics Market is experiencing significant growth due to the increasing trend towards personalized medicine in various therapeutic areas, including cancer, cardiovascular diseases, neurological disorders, and infectious diseases. The use of genetic sequencing and genomics in drug development is driving the demand for companion diagnostics. These tests help in identifying biomarkers and selecting the right patient population for specific therapies, such as immunotherapies like CAR T-cell treatment and immunotherapies, including checkpoint inhibitors. Next-generation sequencing (NGS) plays a crucial role in tumor genome analysis, enabling the identification of molecular targets for therapeutic medications. Immunohistochemistry (IHC) is another essential diagnostic tool used in cancer diagnosis and therapy selection. The market is witnessing innovation in the field of personalized medicine, with companies like Gilead Sciences leading the way in developing companion diagnostics for chronic diseases such as lung cancer and hepatitis B. The ongoing clinical trials for SARS-CoV-2 variants and therapeutic medications also present opportunities for the market. The market caters to various therapeutic areas, including cancer, neurological disorders, cardiovascular diseases, and inflammatory diseases. Companion diagnostics provide molecular data to aid in therapy choices, enabling effective disease treatments and improving patient outcomes. The market is expected to continue growing as the demand for personalized medicine and cutting-edge sequencing technologies increases.
The incidence of breast cancer in the US is on the rise, particularly among women, due to unhealthy lifestyle choices such as poor diets, lack of physical activity, tobacco use, and excessive alcohol consumption. According to the CDC, approximately 250,000 breast cancer cases are diagnosed annually among women in the US. This increasing number of breast cancer cases leads to a corresponding rise in the number of women undergoing cancer treatment, which includes chemotherapy, hormonal therapy (Arimidex, Aromasin, Evista, Fareston), and radiation. Companion diagnostics play a crucial role in these treatments by helping healthcare providers personalize treatment plans based on individual patient characteristics. With the growing need for accurate and efficient cancer diagnosis and treatment, the demand for companion diagnostics is expected to increase significantly.
Request Sample of our comprehensive report now to stay ahead in the AI-driven market evolution!
Market Challenges
- The Companion Diagnostics Market is experiencing significant growth due to the increasing importance of personalized medicine in various therapeutic areas, including cancer, cardiovascular diseases, neurological disorders, and infectious diseases. Drug development in these areas relies heavily on genetic sequencing and genomics to identify biomarkers for patient-selection diagnostic frameworks. Next-generation sequencing (NGS) plays a crucial role in analyzing the tumor genome and identifying mutations for targeted therapies. Cancer treatments, such as CAR T-cell therapy and immunotherapies, require precise diagnosis and monitoring. Immunohistochemistry (IHC) and ISH are essential tools for diagnosing and monitoring cancer, while SARS-CoV-2 variants require quick and accurate diagnostic tests. Reference laboratories play a vital role in providing molecular data for therapy choices, including chemotherapy, radiation treatment, and personalized immunotherapies. The market for companion diagnostics is expanding to include chronic diseases like lung cancer and hereditary sequencing for breast cancer. Medications like Gilead Sciences' therapeutic medications require companion diagnostics for optimal effectiveness. The challenges of drug development and clinical trials necessitate the use of cutting-edge sequencing technologies and personalized medicine approaches. The market for companion diagnostics is poised for growth in various therapeutic areas, including malignant growth treatment, personalized immunotherapies, and inflammatory diseases.
- The companion diagnostics market is a competitive industry, with major players holding significant market share. New entrants face challenges due to high product development costs and the financial strain of limited capital and small-scale manufacturing. Established players, however, are well-positioned, continually innovating and expanding their manufacturing facilities globally, particularly in developing countries. These strategic moves enable them to offer advanced, efficient, and safer companion diagnostics to meet growing demand.
Discover how AI is revolutionizing market trends- Get your access now!
Segment Overview
This companion diagnostics market report extensively covers market segmentation by
- 1.1 Life science
- 1.2 Health centers
- 1.3 Others
- 2.1 Oncology
- 2.2 Neurology
- 2.3 Others
- 3.1 North America
- 3.2 Europe
- 3.3 Asia
- 3.4 Rest of World (ROW)
1.1 Life science- The Companion Diagnostics Market is witnessing significant growth due to the increasing need for pharmaceutical and biotechnology companies to develop high-quality, regulatorily compliant drugs and therapeutic treatments. During the drug discovery process, companion diagnostic products are utilized in the preclinical and clinical stages to evaluate drug response biomarkers, toxicity, and immunotherapy response. These diagnostics play a crucial role in drug discovery by testing multiple targets in a single reaction, reducing the number of samples and hands-on time required, and generating comprehensive data about each analyte of interest. With the high cost of developing and commercializing drugs (over USD2 billion), pharmaceutical and biotechnology companies cannot afford losses if a drug fails. Companion diagnostics help determine the elemental composition and impurity quantification, minimizing drug failures. The prime goal is to accelerate the drug discovery process by screening compound libraries for drug lead identification and optimization. Partnerships with contract research organizations (CROs) and medical device manufacturers are also driving the adoption of companion diagnostics. These factors are expected to fuel the growth of the life science segment of the global Companion Diagnostics Market throughout the forecast period.
Download a Sample of our comprehensive report today to discover how AI-driven innovations are reshaping competitive dynamics
Research Analysis
The Companion Diagnostics Market is a rapidly growing sector in the healthcare industry, focused on developing diagnostic tests that help select the most effective therapy choices for patients based on their molecular data. These tests play a crucial role in drug development, particularly in cancer treatment, where genetic sequencing and genomics are used to identify biomarkers and patient-selection diagnostic frameworks. Next-generation sequencing (NGS) is a cutting-edge technology used to analyze a patient's tumor genome, enabling the development of targeted therapeutic medications, including immunotherapies like CAR T-cell therapy. The market spans various disease areas, including cancer, chronic diseases such as neurological disorders and cardiovascular diseases, and hereditary sequencing. Companies in this market provide patient-choice diagnostic systems, helping ensure that patients receive the most appropriate disease treatments based on their unique molecular profiles.
Market Research Overview
The Companion Diagnostics Market refers to in vitro diagnostic tests that are designed to identify specific biomarkers or genetic mutations in patients, guiding therapy choices for effective disease treatments. These tests play a crucial role in drug development, particularly in cancer, where they aid in patient selection using diagnostic frameworks based on molecular data from tumor genomes. Technologies such as next-generation sequencing (NGS), immunohistochemistry (IHC), and hereditary sequencing are used to identify biomarkers in various therapeutic areas, including cancer, neurological diseases, cardiovascular diseases (CVDs), and infectious diseases. Companion diagnostics are essential for personalized medicine, enabling the selection of targeted therapeutic medications, such as immunotherapies like CAR T-cell therapy, and traditional treatments like chemotherapy and radiation. In the context of cancer, these tests are critical for guiding therapy choices for malignant growth treatments, including immunotherapies and targeted therapies. In the era of personalized medicine, companion diagnostics are becoming increasingly important for selecting the most effective therapy for each patient, based on their unique genetic makeup and disease characteristics. The market for companion diagnostics is expected to grow significantly due to the increasing focus on personalized medicine, the development of cutting-edge sequencing technologies, and the expanding range of therapeutic areas, including cancer, neurological disorders, cardiovascular diseases, and inflammatory diseases. The market also includes reference laboratories and biotechnology companies that specialize in the development and commercialization of companion diagnostics. In the context of cancer, companion diagnostics are playing a pivotal role in the development of new therapies, such as immunotherapies and targeted therapies, and are essential for guiding therapy choices for patients with various types of cancer, including breast cancer, lung cancer, and others. The ongoing COVID-19 pandemic has also highlighted the importance of companion diagnostics in the development of therapeutic medications for SARS-CoV-2 and identifying variants of the virus. Overall, the companion diagnostics market is a dynamic and rapidly evolving field, driven by advances in genomics, biomarker discovery, and personalized medicine.
Table of Contents:
1 Executive Summary
2 Market Landscape
3 Market Sizing
4 Historic Market Size
5 Five Forces Analysis
6 Market Segmentation
- End-user
- Life Science
- Health Centers
- Others
- Indication
- Oncology
- Neurology
- Others
- Geography
- North America
- Europe
- Asia
- Rest Of World (ROW)
7 Customer Landscape
8 Geographic Landscape
9 Drivers, Challenges, and Trends
10 Company Landscape
11 Company Analysis
12 Appendix
About Technavio
Technavio is a leading global technology research and advisory company. Their research and analysis focuses on emerging market trends and provides actionable insights to help businesses identify market opportunities and develop effective strategies to optimize their market positions.
With over 500 specialized analysts, Technavio's report library consists of more than 17,000 reports and counting, covering 800 technologies, spanning across 50 countries. Their client base consists of enterprises of all sizes, including more than 100 Fortune 500 companies. This growing client base relies on Technavio's comprehensive coverage, extensive research, and actionable market insights to identify opportunities in existing and potential markets and assess their competitive positions within changing market scenarios.
Contacts
Technavio Research
Jesse Maida
Media & Marketing Executive
US: +1 844 364 1100
UK: +44 203 893 3200
Email: media@technavio.com
Website: www.technavio.com/
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/companion-diagnostics-market-to-grow-by-usd-28-98-billion-2024-2028-driven-by-personalized-medicine-adoption-with-ai-powering-market-evolution---technavio-302320989.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/companion-diagnostics-market-to-grow-by-usd-28-98-billion-2024-2028-driven-by-personalized-medicine-adoption-with-ai-powering-market-evolution---technavio-302320989.html
SOURCE Technavio